
Does Kinetic Sand Help with Anxiety? Exploring Therapeutic Effects
Explore whether kinetic sand helps with anxiety, including sensory benefits, calming effects, and practical tips for safe play.
#1 Toys Manufacturer in China. WhatsApp: +86 180-0088-4063. Email: [email protected]
#1 Toys Manufacturer in China. WhatsApp: +86 180-0088-4063. Email: [email protected]
If we only use magic sand as a toy, it would be a waste. In fact, it is more useful than you think. You may feel unfamiliar and find it difficult to understand “hydrophobicity” and “non-polarity”. But in science classes, teachers will use magic sand to help students understand these abstract physical and chemical concepts.
Now, let’s feel the “magic” of this Magic Sand.
At first glance, Magic Sand looks like regular beach sand, except for its color. However, when you dip the two types of sand into water, you’ll notice that they behave completely differently.

In water, magic sand will condense into clumps or pillars. Let’s look closely, you will find that the surface of the "sand pillars" has a transparent film, which sometimes looks white. On the other hand, beach sand will fall to the bottom in a scattered manner, and when you stir it, it will make the water turbid.
Then, we take the two types of sand out of the water, and something magical happens:
The moment it leaves the water, the film on the surface of the magic sand disappears, revealing the dry sand inside. Beach sand is still wet even after leaving the water for a while.
Sometimes, we mistakenly believe that sand dries up immediately after leaving the water, although the fact is that this magic sand never gets wet. Everything looks like some kind of magic, but this is both scientific and common in our lives.
A plant that grows in a pond has the same magic, it is the lotus leaf.
Perhaps different from what you think, plants do not want rainwater to stay on their leaves for a long time. Therefore, they have evolved strange shapes to prevent rainwater from accumulating. For example, in the rainforest, some leaves have long pointed tails, and some leaves have many holes.
The lotus leaves that live on the water take a more direct approach, they repel water. When rain falls on the lotus leaves, the drops roll off like beads and will not stay on the leaves. And if you immerse the lotus leaves in water, you will see the same scene as the magic sand: the lotus leaves also have a transparent film on the surface, and they dry the moment they leave the water.
Thanks to the repelling of water, lotus leaves are almost never rotten and dirty (without water, dust and bacteria have nowhere to settle).
This self-cleaning property is also known as the lotus effect – as rainwater flows away, it picks up dust and makes the lotus clean.
Lotus leaves repelling water is a special case. In nature, most materials are hydrophilic(“like water”). Such as the sand on the beach, it attracts water molecules. So when the tide rises, the sand will be wetted by the sea water.
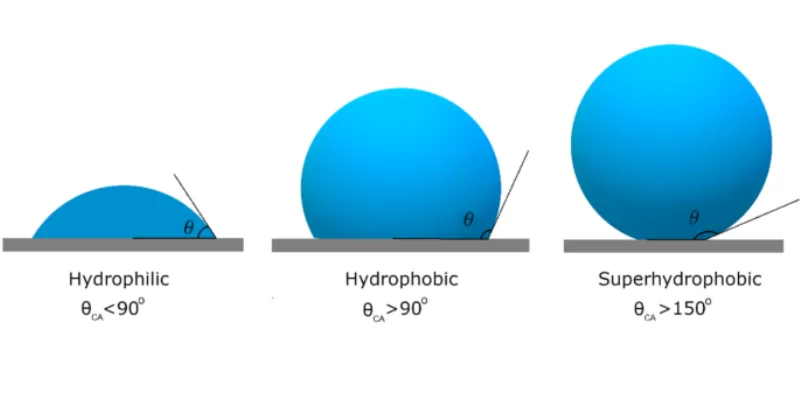
The opposite property of hydrophilicity is hydrophobicity, or “water-fearing”.
From the above comparative experiment, we find that magic sand in water forms a thin air film. This is because the surface of magic sand is hydrophobic, and it will contact water as little as possible. When poured into the water, the sand will bring in more air, which forms a film on the surface.
At present, there are two ways to make hydrophobic magic sand. The simplest is to cover the surface of the sand with hydrophobic substances.
Wax, resin, and plastic are common hydrophobic materials. When you drop water on a waxy surface, the water drop forms a bead instead of spreading out. The secret of the lotus leaf’s super-hydrophobicity is the wax crystal structure on its surface.
Magic sand can be made by covering the surface of ordinary sand with hydrophobic materials. For example, you can spread sand in a tray and evenly spray it with a waterproof coating. After waiting for a while, pour the sand into the water and see what happens.
The reason hydrophobic substances repel water is that polar molecules and nonpolar molecules. In chemistry, molecules are called polar when one side of the molecule has an electric charge that doesn’t cancel each other out.
Polar molecules have uneven charges, and water is a classic example. Its chemical formula is H2O, with two hydrogen atoms and one oxygen atom. The oxygen atom strongly attracts the hydrogen atoms’ electrons, bringing them closer to the oxygen nuclei. As a result, the hydrogen atoms in water have slightly positive charges, while the oxygen atom has slightly negative charges.
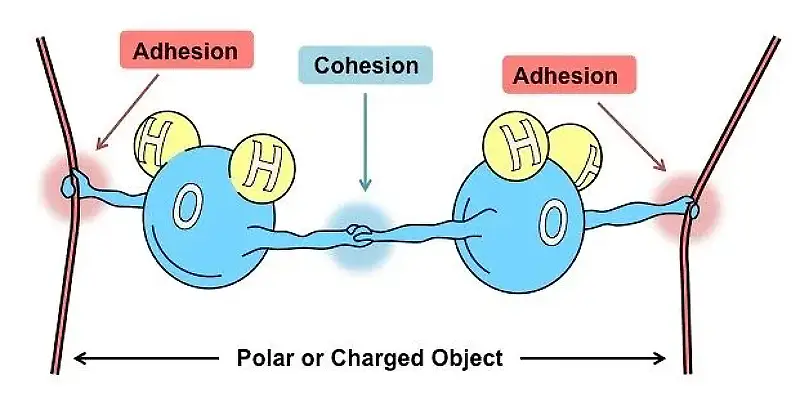
We know that opposite charges attract each other. Therefore, polar molecules (like regular beach sand) tend to stick together and dissolve easily in water. Nonpolar molecules, on the other hand, have an even distribution of electrons, or symmetrical polar bonds, and their charges cancel each other out.
Nonpolar molecules (like wax) are generally insoluble in water. They prefer other nonpolar molecules to water molecules. So, when Magic Sand is submerged in water, a film of air appears – air is generally nonpolar. Magic Sand prefers to be with air rather than water.
Another way to manufacture magic sand is to use trimethylsilanol ((CH 3 ) 3 SiOH) or trimethylchlorosilane ((CH 3 ) 3 SiCl). It forms a nano-thin coating of silicon compounds on the surface of ordinary sand.
The nano-coating is very thin (one billionth of a meter) and cannot be observed by our eye, but it is what gives ordinary sand the “magic” of super hydrophobicity.
In fact, the microscopic world is more wonderful than we think. Under a high-power microscope, we can see that there are many uneven papillary structures on the surface of lotus leaves. They are very small, with a diameter of only 5-15 microns and a height of 1-20 microns. When it rains, water droplets actually only come into contact with the tips of these smiling papillae, greatly reducing the contact area between water and lotus leaves.

In daily life, the surface of non-stick pans also has a thin layer of nano-coating, and its principle is similar to that of magic sand.
From the Material Safety Data Sheet (MSDS) for Magic Sand, we can see that it is mainly composed of silica sand (sometimes up to 99%), also known as quartz.
Quartz sand is found on most beaches around the world. Its chemical name is silicon dioxide. The nano-coating that makes it hydrophobic – silicone compounds – actually comes from the sand itself.
The content of pigment or colorant in Magic Sand is tiny, usually only 0.4%. However, it is because of this that Magic Sand has a variety of bright colors.
Although the ingredients of Magic Sand are simple, its uses and benefits go far beyond playing and teaching.
The origin of hydrophobic sand comes from an idea for oil spill control.
Since oil is lighter than water, oil spills in oceans or rivers affect large areas of water and are difficult to clean up. Hydrophobic sand is a clever idea as it attracts oil and repels water. So, we could spread hydrophobic sand on the surface of the oil spill, and the sand will absorb the oil and form large heavy balls to sink into the water.
Cleaning up these hydrophobic balls is much easier than dealing with the oil directly. However, this idea has a fatal flaw: it is too costly. Thus, in practice, people have not realized the use of hydrophobic sand to control oil spills.
In cold winters, water in the soil will freeze. As water turns to ice, it expands in volume, which often causes cracks and damage. Therefore, when building foundations, people add hydrophobic sand to form a waterproof layer.
Hydrophobic magic sand prevents rainwater from seeping into the soil and buildings. And on the other hand, magic sand does not freeze even in extremely cold places. When people need to repair underground buildings or pipes in such a climate, they can easily dig up the magic sand.
Theoretically, magic sand can last forever.
But if the coating on the surface of the magic sand is damaged, it will become ordinary sand and no longer have the “magic” of hydrophobicity. To make the magic sand last longer, we should avoid it contacting hard objects to reduce the wear of the coating.
Moreover, do not let the magic sand soak in open water for a long time. The sand will not go bad, but the water will breed mosquitoes and bacteria.
A play tip is not to mix with magic sand of different colors, as you may never be able to separate them again.
Magic sand is insoluble, so it is best not to pour it down the drain with water. The sand may accumulate somewhere and eventually cause a clog.
It is easy to separate the magic sand from the water with a towel or a small fishing net, and then put the dry magic sand back into the sealed bag or box.
Using magic sand for science experiments and teaching is a common practice in schools. This educational toy allows children and students to learn complex physics and chemistry while playing.
But magic sand has more uses. In industry, people once wanted to use it to clean oil spills on the water. Although it was too expensive to do so, people did not give up on it. In cold places, people use hydrophobic sand to protect underground buildings and pipelines.
Maybe today’s children just put magic sand in water to observe its properties. But in the near future, they will apply superhydrophobic materials and nano-coatings to wider fields.
It all starts with the magic sand.
References
Video Source
Yes, Magic Sand is fine and does not get wet, so you can vacuum it. And you don't have to worry about the sand clogging your pipes. Moreover, if Magic Sand gets on your carpet, don't use water or a wet towel, as that will only make the situation worse. You can shake the carpet to make the sand off onto a smooth surface, and then collect it with a broom.
However, you can't use a vacuum to deal with Magic Sand in water. Because the vacuum will absorb water, which may damage the machine. The best way to collect Magic Sand in water is to use a small fishing net. This net has small holes that can filter water and keep the dry sand on it.
At last, put the collected magic sand in a sealed box or bag, and you can reuse it next time.
The essence of magic sand is fine sand. Its hydrophobicity is a physical property, not a chemical reaction, so it is safe. However, do not eat or inhale the magic sand. Our bodies cannot process sand, and eating or inhaling it will harm our esophagus, gastrointestinal tract and lungs. And of course, keep it away from your eyes.
When using magic sand, be sure to follow safety tips and play with an adult!
To be honest, yes, magic sand may make a mess. Because of its hydrophobic nature, magic sand does not clump together. Plus, its small particles and light weight make it easy to become messy in the air. So, when using magic sand at home or in the classroom, it is better to put it in water or a sealed box rather than let it sit in the air.
However, if you accidentally spill magic sand on the floor, or worse, on your carpet, just vacuum it up.
In theory, children over 3 years old can play with magic sand. However, at present, magic sand is more common in science classes in senior grades.
Teachers use magic sand to teach students to understand physics and chemistry knowledge such as nanotechnology, surface energy, and molecular interactions. When playing magic sand at home, parents can play the role of teachers and explain the principle of hydrophobicity to their children.
Moreover, we can also use magic sand to create in the water, such as building sand castles, painting, writing, etc. Actually, magic sand is suitable for all ages, except infants and toddlers under 3.
Although both Magic Sand and Kinetic Sand are “sand,” they are different. Simply put, Magic Sand doesn’t get wet and Kinetic Sand doesn’t dry out.
However, they are both great educational toys that teachers love to use in their classrooms. Magic Sand shows older children the characteristics of molecular bonding and polarity. Kinetic Sand, on the other hand, is great for sensory play and hands-on creations for younger kids.
In fact, magic sand is often made in different colors, such as red, yellow, blue, green, etc. Usually, the pigment does not affect the hydrophobicity, so it allows people to customize magic sand of different colors.
However, the color of magic sand in water and in the air will be slightly different. When we put magic sand in water, the surface of the sand looks like a white film due to the hydrophobic effect. Coupled with the refraction of water and glass walls, the color of the sand we see is usually darker than in the air. And the deeper the glass tank, the darker the color of the sand entering the water will be, because the light at the bottom is weaker.
In short, objectively, the color of magic sand depends on the pigment used in its manufacture. But subjectively, by different environments, uses, and observation methods, we may see sand of different shades.
More Related...

Explore whether kinetic sand helps with anxiety, including sensory benefits, calming effects, and practical tips for safe play.

Key differences between kinetic sand and normal sand, including texture, safety, and sensory benefits for children’s play and learning.

Explore what sensory sand is made of, why children love it, and how its unique materials enhance learning, creativity, and fine motor skills.

Discover what makes kinetic sand so special — from its unique texture to the fascinating science behind its magical movement.

Our team will answer your inquiries within 48 hours.
Copyright © 2026 GuangDong AKIA Technology Co,. Ltd. All Rights Reserved